Focus Areas
All Solid State Batteries
Employing solid-state electrolytes (SEs) is a path toward greater battery safety since most inorganic SEs are nonflammable or have much higher ignition temperatures than organic-based electrolytes. Moreover, the integration of lithium metal in all-solid-state batteries (ASSBs) offers the potential for improved specific energy, reaching up to 400 Wh kg−1 at cell-level. However, ASSBs face several critical challenges, including interface stability, dendrite propagation, and the formation of cracks and voids during operation. These issues must be meticulously addressed to realize the full potential of ASSBs
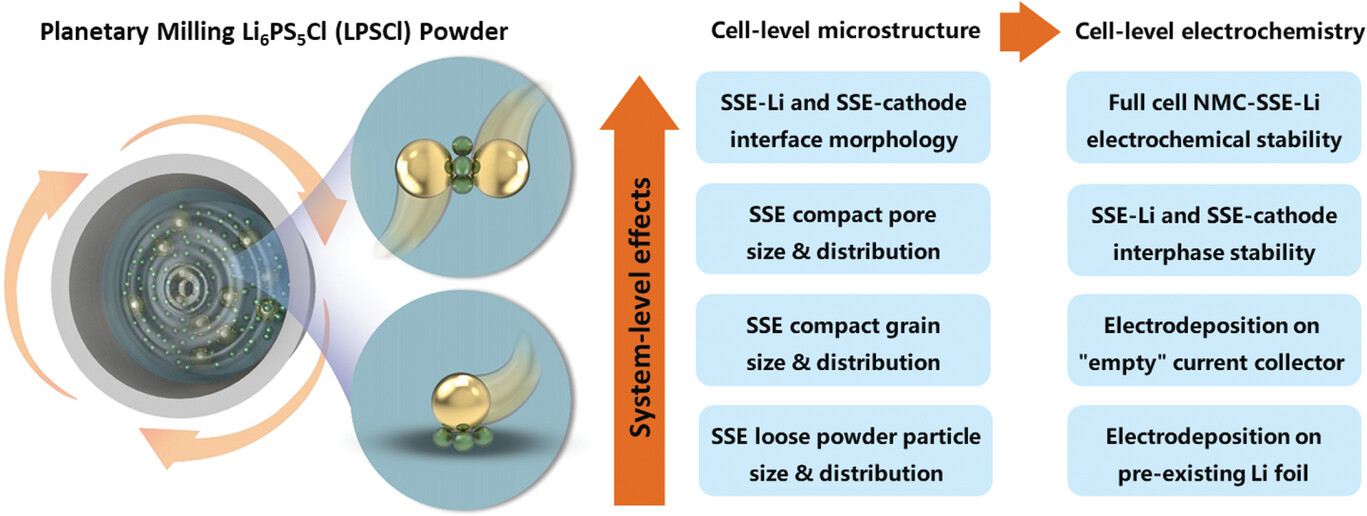
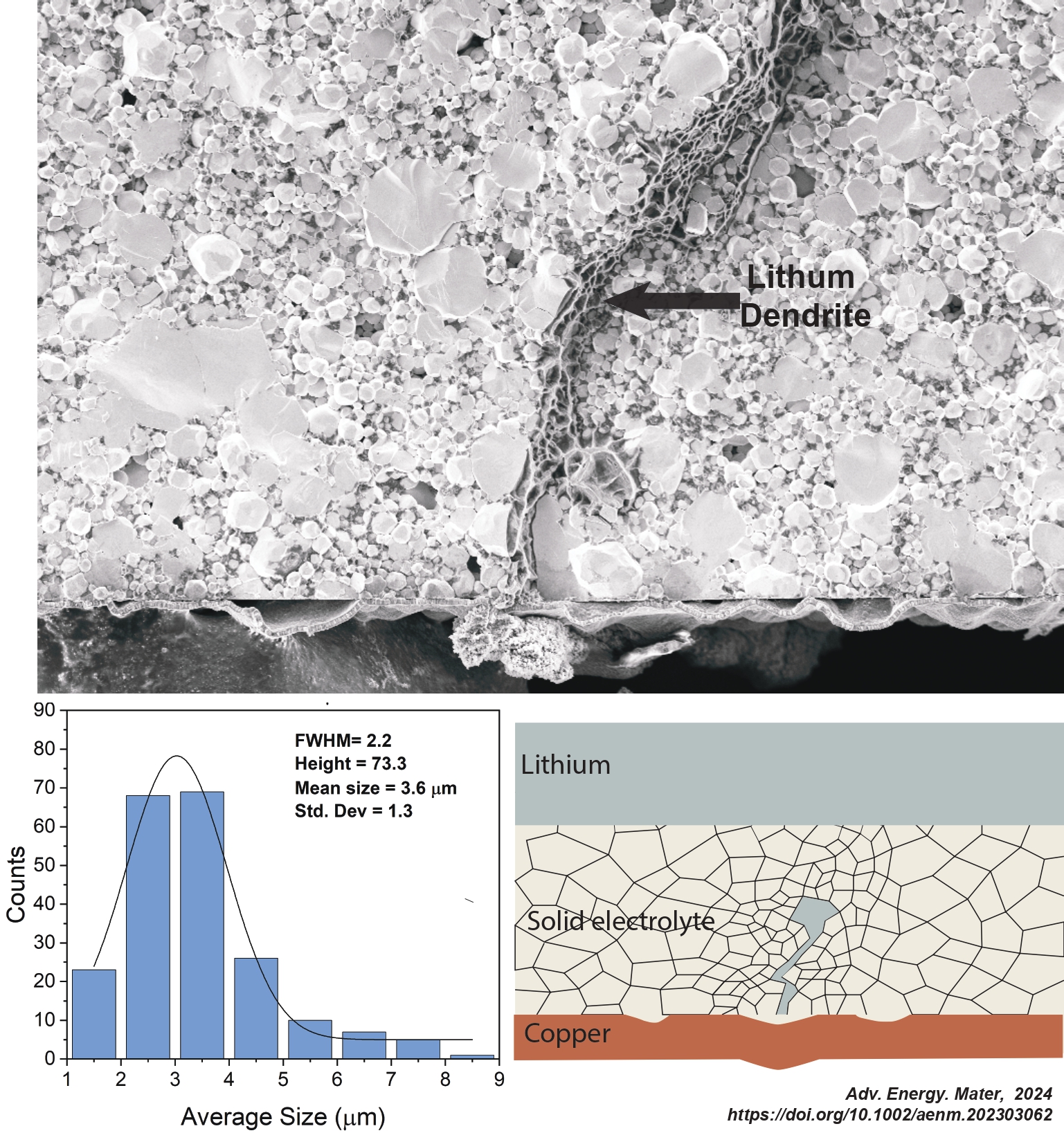
In the article “Mechanical Milling – Induced Microstructure Changes in Argyrodite LPSCl Solid-State Electrolyte Critically Affect Electrochemical Stability”, we combine site-specific cryo-FIB analysis, quantitative stereology, and mesoscale modeling to further elucidate how the microstructure of argyrodite SSE affects electrochemical behavior. Planetary mechanical milling of commercially purchased argyrodite powder in wet media is employed to progressively refine the particle size/distribution as a function of milling time. This results in a reduction of SSE compact grain size/distribution and pore size/distribution. It is shown that an optimized SSE microstructure enables state-of-the-art electrochemical performance without the need for any artificial SEI layers or other secondary modifications. Moreover, analysis of half cells with bilayer electrolytes demonstrates that microstructure directly in contact with the current collector determines stability. For the first time, a lithium metal dendrite that has caused a short circuit in LPSCl is directly imaged at high resolution. This is achieved by cryo-FIB cross–sectioning of a failed SSE taken from a 1.5 mm diameter “mini” symmetrical cell. Site-specific analysis highlights that the lithium metal dendrite has the following features: a) a sheet-like morphology with branching sections; b) traverses the compact intergranularly, moving around large grains rather than through them; c) fills the interparticle voids and reacts with the contacting SSE to form reduction decomposition products, i.e. the dendrite is surrounded by an SEI. It is also demonstrated that the extensively milled microstructures promote uniform early-stage electrodeposition on foil collectors, as well as a more stable SEI. Mesoscale modeling takes these results into account and provides a comprehensive chemomechanical phenomenology of how the identified features of the Li-SSE interface microstructure promote dendritic versus planar growth.
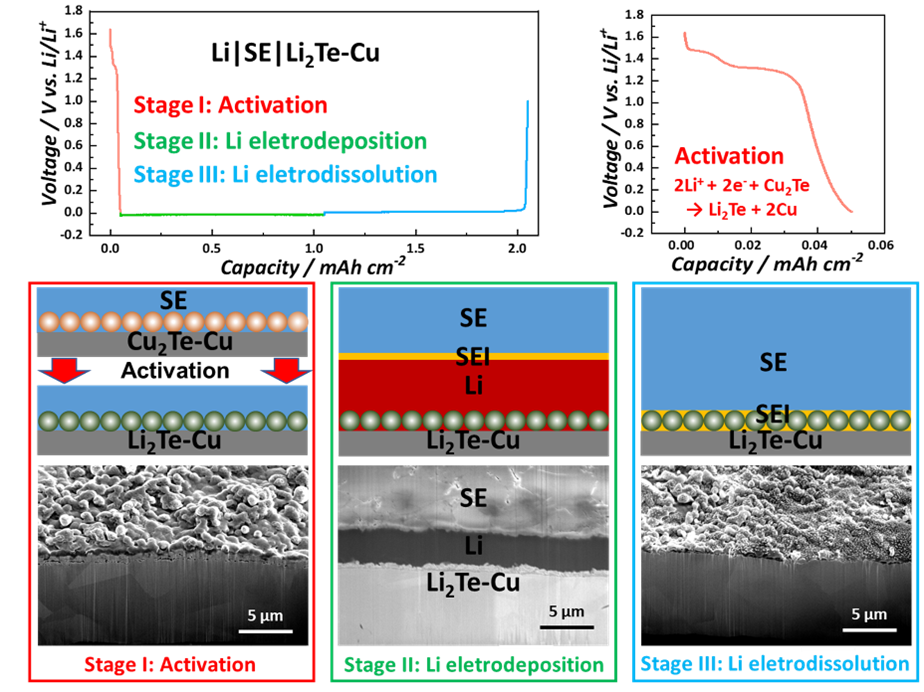
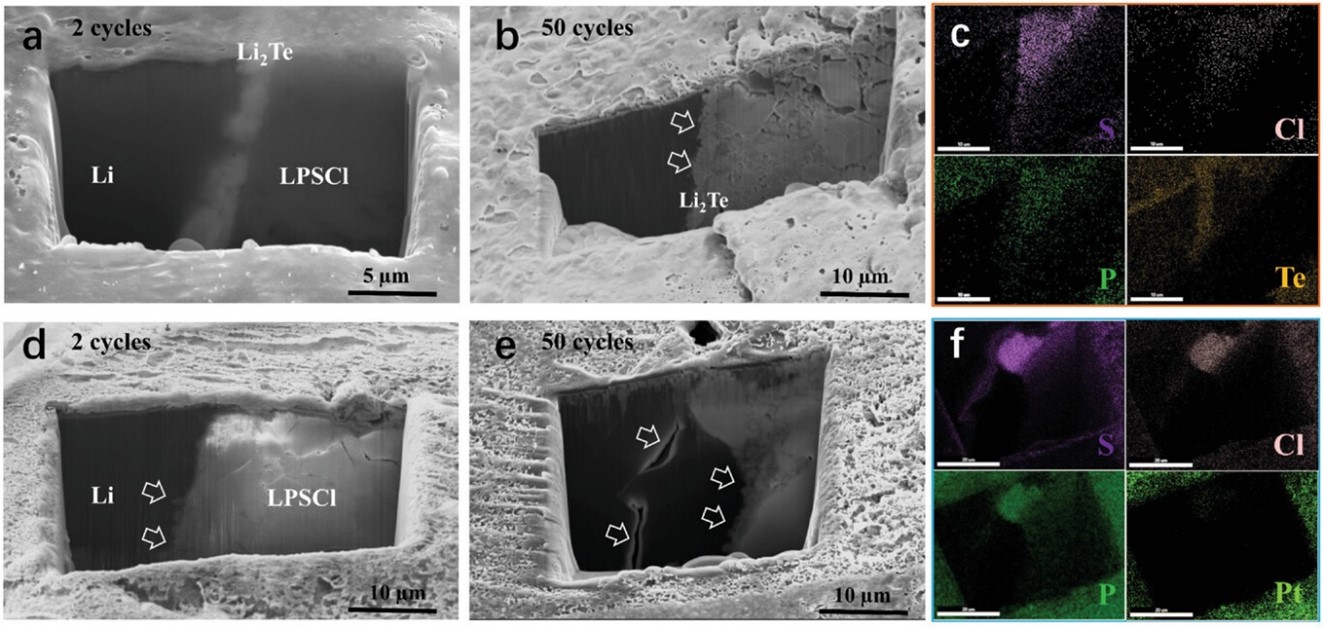
 In the article “Stable Anode-Free All-Solid-State Lithium Battery through Tuned Metal Wetting on the Copper Current Collector”, we report an Anode Free-ASSB (AF-ASSB) employing a sulfide-based SE (argyrodite LPSCl). 1 μm Lithiophilic Li2Te coating on standard planar copper current-collector significantly reduces electrodeposition/electro-dissolution overpotentials and improves CE. During continuous plating experiments using half-cells (1 mA cm−2), the accumulated thickness of electrodeposited Li on Li2Te–Cu is more than 70 μm, which is the thickness of the Li foil counter-electrode. The NMC811 anode-free cell (external pressure 13 MPa) delivers an initial CE of 83% at 0.2C, with a steady-state cycling CE above 99%. Cryo-FIB sectioning demonstrates uniform electrodeposited metal microstructure, with no signs of voids or dendrites at the collector-SE interface. Cryo-FIB also demonstrates that electro-dissolution is uniform and complete, the lithiophilic coating remaining adherent on the collector. By contrast, a bare Cu collector promotes inhomogeneous Li electrodeposition/electro-dissolution, electrochemically inactive “dead metal,” dendrites that extend into the SE, and extensive non-uniform SEI interspersed with pores. DFT and mesoscale calculations consider the thermodynamic stability of lithium atoms versus lithium clusters on Li2Te and Cu surfaces, including subsurface effects after one monolayer is deposited as well as the nucleation-growth behavior. This work paves the way for viable AF-ASSBs that deliver significantly higher specific energies and cost less than ASSBs which require metal or ion-storing anodes.
In the article “Stable Anode-Free All-Solid-State Lithium Battery through Tuned Metal Wetting on the Copper Current Collector”, we report an Anode Free-ASSB (AF-ASSB) employing a sulfide-based SE (argyrodite LPSCl). 1 μm Lithiophilic Li2Te coating on standard planar copper current-collector significantly reduces electrodeposition/electro-dissolution overpotentials and improves CE. During continuous plating experiments using half-cells (1 mA cm−2), the accumulated thickness of electrodeposited Li on Li2Te–Cu is more than 70 μm, which is the thickness of the Li foil counter-electrode. The NMC811 anode-free cell (external pressure 13 MPa) delivers an initial CE of 83% at 0.2C, with a steady-state cycling CE above 99%. Cryo-FIB sectioning demonstrates uniform electrodeposited metal microstructure, with no signs of voids or dendrites at the collector-SE interface. Cryo-FIB also demonstrates that electro-dissolution is uniform and complete, the lithiophilic coating remaining adherent on the collector. By contrast, a bare Cu collector promotes inhomogeneous Li electrodeposition/electro-dissolution, electrochemically inactive “dead metal,” dendrites that extend into the SE, and extensive non-uniform SEI interspersed with pores. DFT and mesoscale calculations consider the thermodynamic stability of lithium atoms versus lithium clusters on Li2Te and Cu surfaces, including subsurface effects after one monolayer is deposited as well as the nucleation-growth behavior. This work paves the way for viable AF-ASSBs that deliver significantly higher specific energies and cost less than ASSBs which require metal or ion-storing anodes.
In the article “Dendrite Growth—Microstructure—Stress—Interrelations in Garnet Solid-State Electrolyte”, tantalum-doped LLZO with a nominal composition of Li6.4La3Zr1.4Ta0.6O12 was fabricated based on two approaches, one involving a conventional route of powder processing followed by reactive sintering, and one incorporating an intermediate-stage high-energy milling step. These fabrication routes resulted in distinct microstructures and associated electrochemical behavior during extended cycling. The relation between the two was then analyzed in detail using surface science and bulk techniques, as well as quantitative stereology. For the first time it is demonstrated that compact grain size distribution critically affects short-circuit failure. Lithium metal dendrites propagate inter granularly along “weak-links” in the microstructure, which are percolating arrays of LLZTO grains that are significantly smaller than the average. Lithium metal also accumulates inside the pores along the dendrite’s path through the compact. These observations point to the role of enhanced electrical conductivity—electron accumulation in the dendrite-driven failure phenomenology. The experimental findings were also complemented by mechanistic modeling to give a comprehensive picture of microstructure–chemo–mechanical stress—electrochemical stability interrelations for garnet-based SSEs.
In the article “Tuned Reactivity at the Lithium Metal–Argyrodite Solid State Electrolyte Interphase”, a tuned reactivity approach is demonstrated, utilizing 2D tellurene as a model system that is dispersed on either the anode or the SSE surface. The dispersed tellurene is not in equilibrium with Li, and upon physical contact reacts to form a thin (0.75 µm) intermetallic bilayer of equilibrium Li2Te and intermediate LiTe3. This bilayer stabilizes the solid electrolyte interphase (SEI) both electrochemically and geometrically. Combined analytical techniques elucidate the kinetic reaction pathway through which the tellurene-modified Li–LPSCl interphase remains intact while the unmodified interphase decomposes. Through Cryo-FIB sectioning and Raman mapping, it is demonstrated that the unmodified interphase is heterogeneous; the reaction layer varying in width and in how far it extends into the electrolyte. There is little evidence for pristine (unreacted) Li filaments/dendrites, implying that such a classical description of electrical short-circuiting may not be fully applicable to highly reactive sulfides. There are large voids in the Li metal anode adjacent to the more extensively reacted regions, while with the Li2Te-LiTe3 interlayer, these voids are absent. This points to heterogenous Li metal-LPSCl reactivity as an additional cause for site-specific Li depletion that results in void formation. With the Li2Te-LiTe3 state-of-the-art electrochemical performance is achieved in symmetric cells, with continuous plating of half-cells, and with an ASSB based on NMC811 cathode. For example, continuous plating of 12 mAh cm−2 on an empty current collector is demonstrated at 0.5 mA cm−2. All-solid-state battery (ASSB) with an NMC811 cathode (9.5 mg cm−2) with a capacity of 154 mAh g−1 at 0.5 C displays 95.4% retention after 400 cycles. Density Functional Theory (DFT) simulations support the above findings, demonstrating enhanced thermodynamic stability and reduced electrical conductivity of the Li2Te–LPSCl interphase. Mesoscale modeling depicts the relationship between interphase heterogeneity and reaction current distribution, signifying the critical role of the Li2Te layer in enabling a uniform reaction front.
Sodium and potassium ion storage

A hard carbon ion insertion anode gives much higher overpotential with K+ vs Na+. This significantly lowers the energy–power of symmetric hybrid potassium ion capacitors (KICs) vs sodium ion capacitors (NICs). However, high surface area, ion adsorption carbon works well with both K and Na, opening the possibility for high performance symmetric KICs. In article “Potassium Ion Storage: Direct Structure–Performance Comparison of All‐Carbon Potassium and Sodium Ion Capacitors“, a direct performance comparison of potassium ion capacitors versus the better‐known sodium ion capacitors has been provided.
Sodium and potassium metal anodes
The metal anode is the essential component of emerging energy storage systems such as sodium/potassium sulfur and sodium/potassium selenium batteries. Greatly improved understanding and control of the SEI structure is the key to cycling stability of Na/K-metal anode. A holistic approach involving complementary post-mortem, in situ, and operando analyses to elucidate full battery cell level structure−performance relations is the need of the hour.

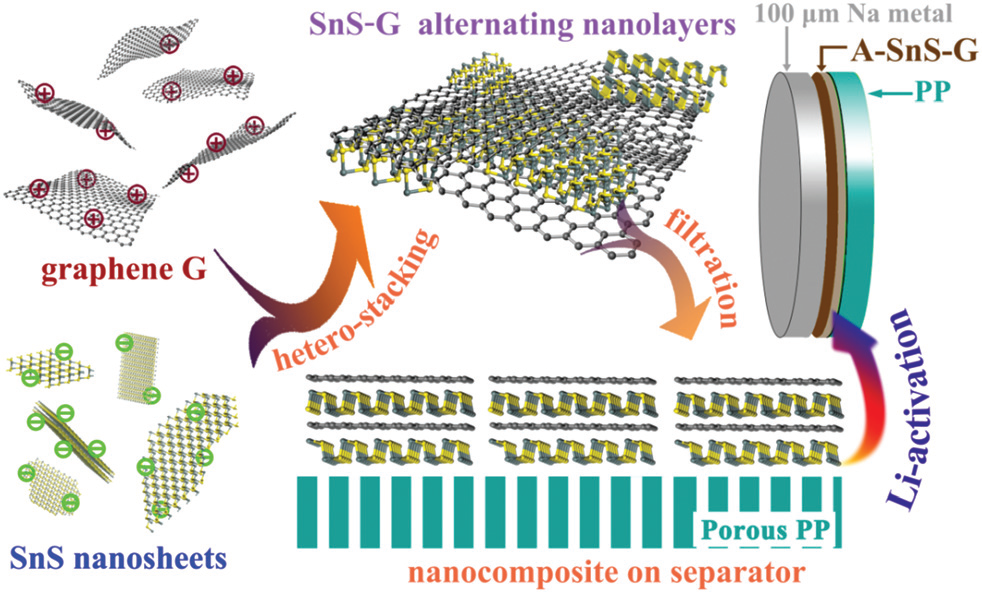
In article “Lithium-activated SnS–graphene alternating nanolayers enable dendrite-free cycling of thin sodium metal anodes in carbonate electrolyte“, Li-ion activated tin sulfide graphene nanocomposite membrane (A-SnS–G) is employed as an artificial SEI layer, allowing cyclability of record-thin 100 mm Na metal foils. A-SnS–G is initially placed onto the polypropylene (PP) separator but becomes in situ transferred onto the Na metal surface. Symmetric metal cells protected by A-SnS–G achieve lowoverpotential extended high-rate cycling in a standard carbonate electrolyte. Accumulated capacity of 1000 mAh/cm2 is obtained after 500 cycles at 4 mA/cm2 , with accumulated capacity-to-foil capacity (A/F) ratio of 90.9. This is among the most favorable cycle life, accumulated capacity, and anode utilization combinations reported. As a proof of principle, an SMB with a high mass loading (6 mg/cm2) NVP cathode and a A-SnS–G protected anode delivered extended cyclability, achieving 74 mA h/g after 400 cycles at 0.4C

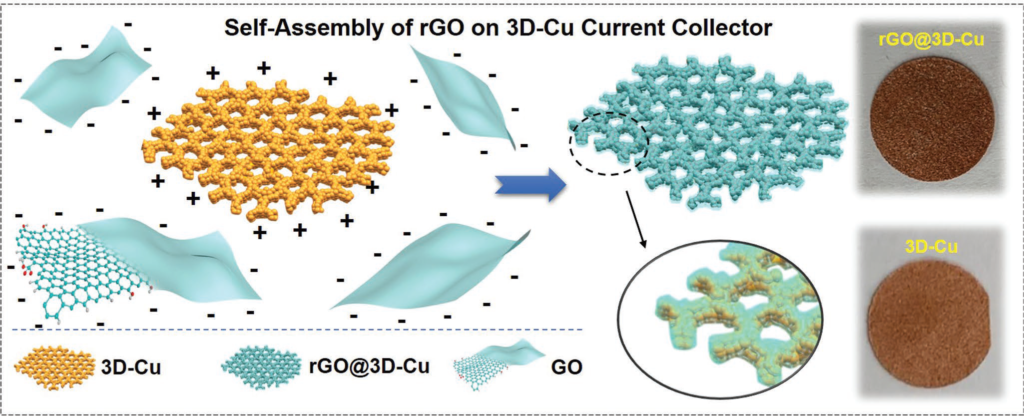
In article “Dendrite-Free Potassium Metal Anodes in a Carbonate Electrolyte“, it is demonstrated that a tailored current collector will stabilize the metal plating–stripping behavior even with a conventional KPF6-carbonate electrolyte. A 3D copper current collector is functionalized with partially reduced graphene oxide to create a potassiophilic surface, the electrode being denoted as rGO@3D-Cu. Potassiophilic versus potassiophobic experiments demonstrate that molten K fully wets rGO@3D-Cu after 6 s, but does not wet unfunctionalized 3D-Cu. Electrochemically, a unique synergy is achieved that is driven by interfacial tension and geometry: the adherent rGO underlayer promotes 2D layer-by-layer (Frank–van der Merwe) metal film growth at early stages of plating, while the tortuous 3D-Cu electrode reduces the current den-sity and geometrically frustrates dendrites. The rGO@3D-Cu symmetric cells and half-cells achieve state-of-the-art plating and stripping performance. The half-cells cells of rGO@3D-Cu (no K reservoir) are stable at 0.5 mA/cm2 for 10,000 min, and at 1 mA/cm−2 for 5000 min.
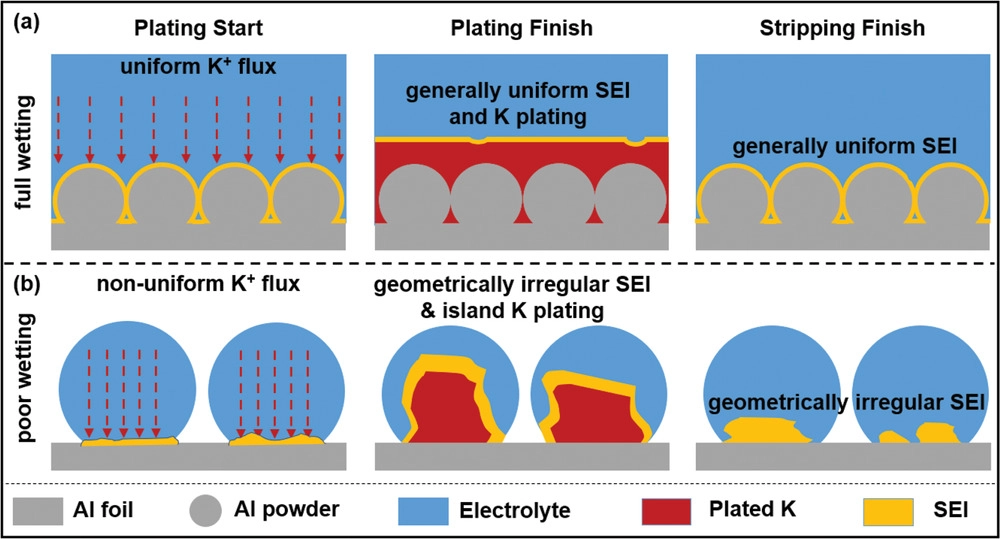
 In article “Stable Potassium Metal Anodes with an All-Aluminum Current Collector through Improved Electrolyte Wetting“, dendrite-free plating/stripping is achieved through improved electrolyte wetting, employing an aluminum-powder-coated aluminum foil “Al@Al,” without any modification of the support surface chemistry or electrolyte additives. The reservoir-free Al@Al half-cell is stable at 1000 cycles (1950 h) at 0.5 mA/cm2, with 98.9% cycling Coulombic efficiency and 85 mV overpotential. The pre-potassiated cell is stable through a wide current range, including 130 cycles (2600 min) at 3.0 mA/cm2, with 178 mV overpotential. Al@Al is fully wetted by a 4 M KFSI/DME electrolyte (θCA = 0°), producing a uniform solid electrolyte interphase (SEI) during the initial galvanostatic formation cycles. On planar aluminum foil with a nearly identical surface oxide, the electrolyte wets poorly (θCA = 52°). This correlates with coarse irregular SEI clumps at formation, 3D potassium islands with further SEI coarsening during plating/stripping, possibly dead potassium metal on stripped surfaces, and rapid failure. The electrochemical stability of Al@Al versus planar Al is not related to differences in potassiophilicity (nearly identical) as obtained from thermal wetting experiments. The key fundamental takeaway is that the incomplete electrolyte wetting of collectors results in early onset of SEI instability and dendrites.
In article “Stable Potassium Metal Anodes with an All-Aluminum Current Collector through Improved Electrolyte Wetting“, dendrite-free plating/stripping is achieved through improved electrolyte wetting, employing an aluminum-powder-coated aluminum foil “Al@Al,” without any modification of the support surface chemistry or electrolyte additives. The reservoir-free Al@Al half-cell is stable at 1000 cycles (1950 h) at 0.5 mA/cm2, with 98.9% cycling Coulombic efficiency and 85 mV overpotential. The pre-potassiated cell is stable through a wide current range, including 130 cycles (2600 min) at 3.0 mA/cm2, with 178 mV overpotential. Al@Al is fully wetted by a 4 M KFSI/DME electrolyte (θCA = 0°), producing a uniform solid electrolyte interphase (SEI) during the initial galvanostatic formation cycles. On planar aluminum foil with a nearly identical surface oxide, the electrolyte wets poorly (θCA = 52°). This correlates with coarse irregular SEI clumps at formation, 3D potassium islands with further SEI coarsening during plating/stripping, possibly dead potassium metal on stripped surfaces, and rapid failure. The electrochemical stability of Al@Al versus planar Al is not related to differences in potassiophilicity (nearly identical) as obtained from thermal wetting experiments. The key fundamental takeaway is that the incomplete electrolyte wetting of collectors results in early onset of SEI instability and dendrites.
Selenium (Se) and Selenium-Sulfur (SeS) cathodes
In article “Selenium-sulfur (SeS) fast charging cathode for sodium and lithium metal batteries“, we report a bifunctional sodium metal battery (SMB) and lithium metal battery (LMB) cathode based on 63 wt.% Selenium-Sulfur covalently bonded to a co-pyrolyzed polyacrylonitrile (PAN) host, termed “SeSPAN”. This dense, low surface area, fully-amorphous electrode offers a highly favorable combination of reversible capacity, rate capability, and cycling life.
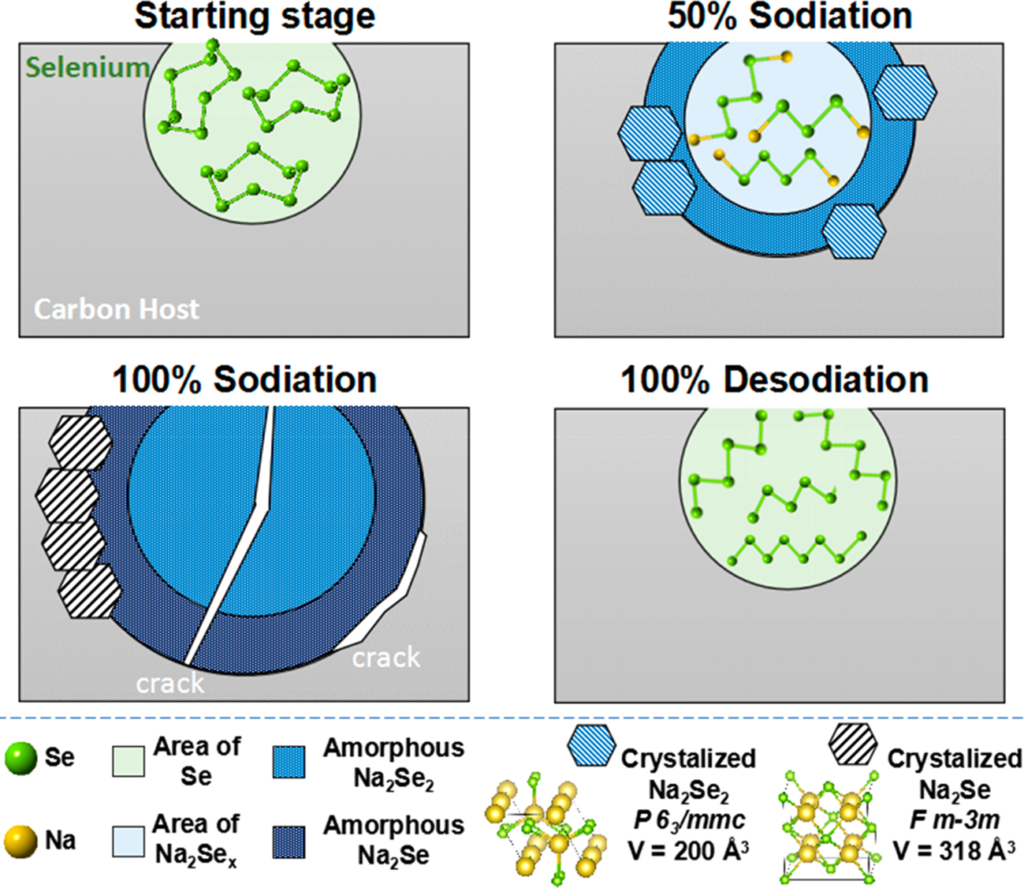
In article “Site-Specific Sodiation Mechanisms of Selenium in Microporous Carbon Host“, We combined advanced TEM (HRTEM, HAADF, EELS) with solid-state (SS)MAS NMR and electroanalytical techniques (GITT, etc.) to understand the site-specific sodiation of selenium (Se) encapsulated in a nanoporous carbon host. The architecture employed is representative of a wide number of electrochemically stable and rate-capable Se-based sodium metal battery (SMB) cathodes. SSNMR demonstrates that during the first sodiation, the Se chains are progressively cut to form an amorphous mixture of polyselenides of varying lengths, with no evidence for discrete phase transitions during sodiation. It also shows that Se nearest the carbon pore surface is sodiated first, leading to the formation of a core–shell compositional profile. HRTEM indicates that the vast majority of the pore-confined Se is amorphous, with the only localized presence of nanocrystalline equilibrium Na2Se2 (hcp) and Na2Se (fcc). A nanoscale fracture of terminally sodiated Na–Se is observed by HAADF, with SSNMR, indicating a physical separation of some Se from the carbon host after the first cycle. GITT reveals a 3-fold increase in Na+ diffusivity at cycle 2, which may be explained by the creation of extra interfaces. These combined findings highlight the complex phenomenology of electrochemical phase transformations in nanoconfined materials, which may profoundly differ from their “free” counterparts.
Inorganics Driven High Temperature Fouling of Metal Surfaces in Oil Refining
 Fouling Reactor Design
Fouling Reactor Design
- 1200 mL crude + 800 mL headspace per run
- Prior to testing, the reactor was sealed, purged with nitrogen and pressurized to 8.84 atm
- During testing the oil was stirred using a stirrer rod rotating at 300 RPM
- Constant current, (not constant T)
- Resistivity (R) of wires vs. T is calibrated in-situ and ex-situ
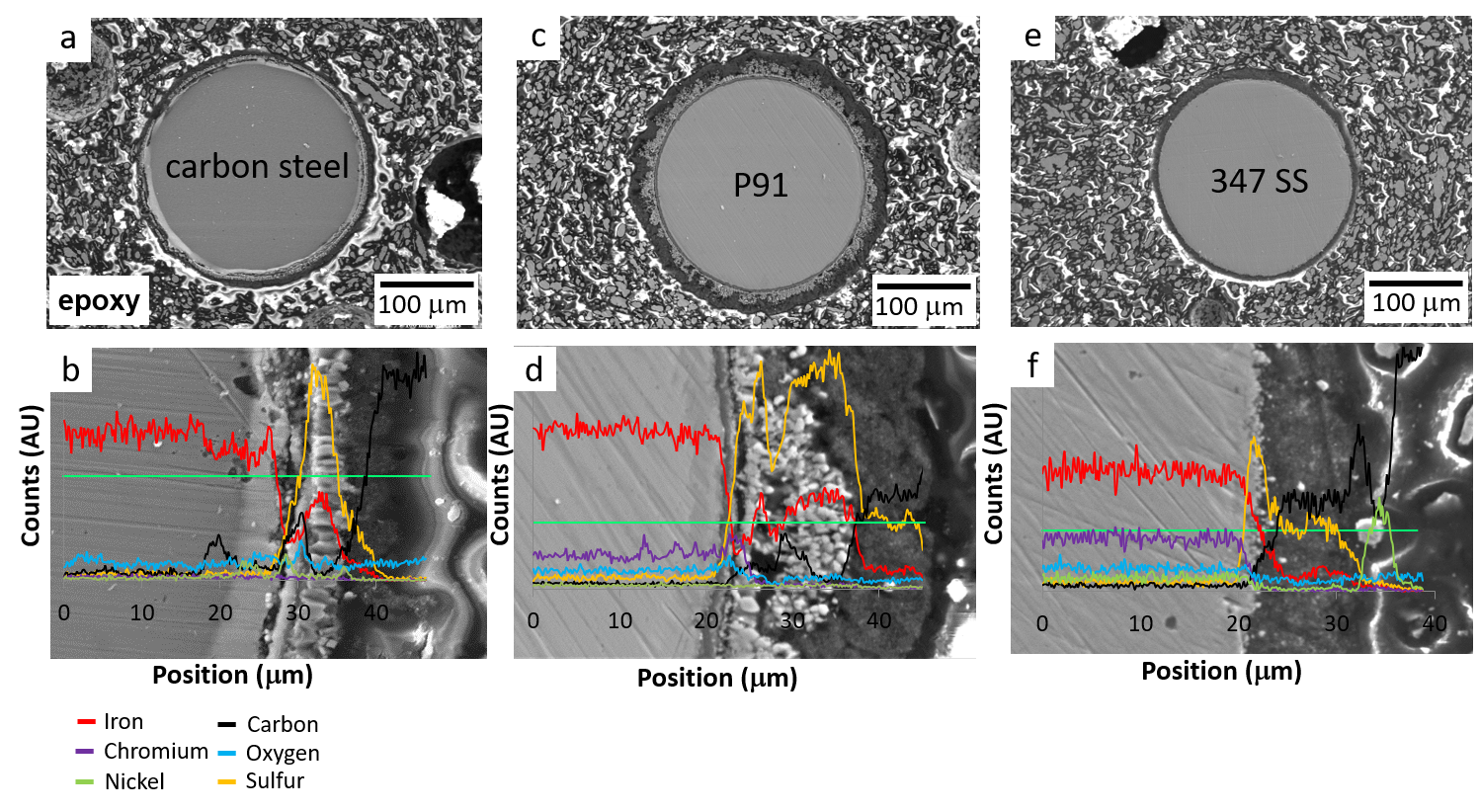
Effect of Metallurgy on Fouling Behavior: In-situ Fouling Factors
Blend B-C. Metallurgy 347 SS, P91, carbon steel, wire T = 490 °C, oil T = 290 °C.
At 60 mins, Fouling P91 > 347 > Carbon Steel
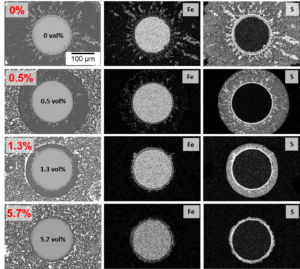
SEM and EDX elemental maps of 316 stainless steel wire cross sections fouled for 1400 minutes, showing the effect of thiophene as an oil additive.
(a) No additive.
(b) 0.5 vol% thiophene.
(c) 1.3 vol% thiophene.
(d) 5.7 vol% thiophene.
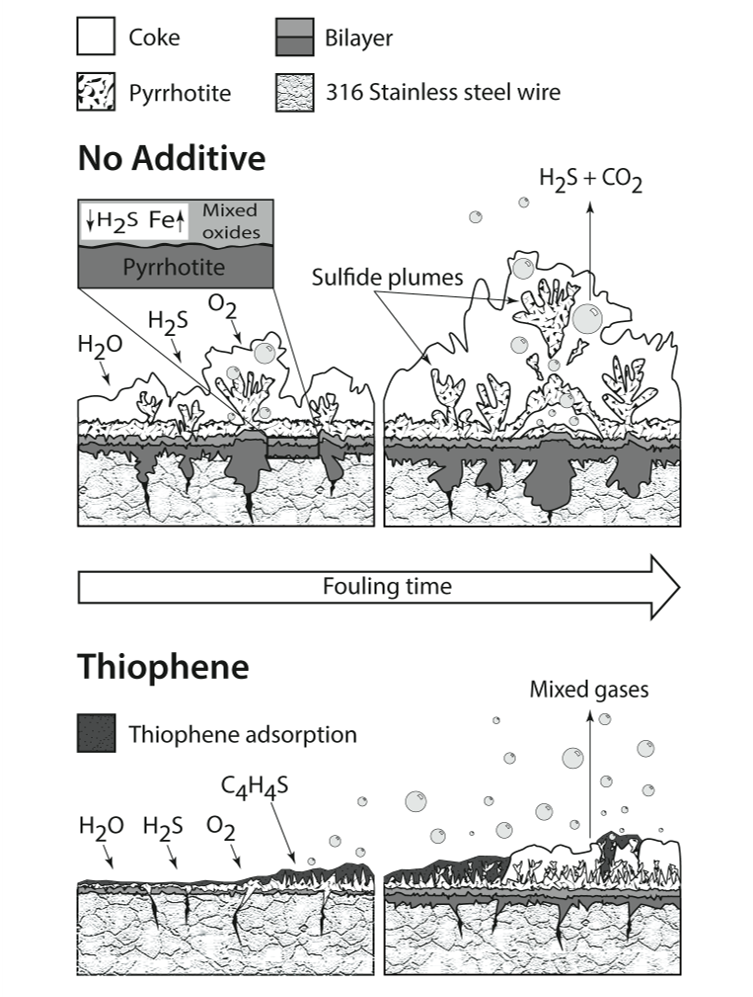
Schematic diagram showing the corrosion fouling with and without thiophene.
- Thiophene inhibits fouling reactions.
- In 316 Metallurgy an inner oxide – sulfide is formed, which presumably reduces H2S ingress and outward diffusion of Fe.
- Sulfide promoted chronic fouling in a refinery: A broad phenomenon spanning a range of heat transfer surfaces and oil types.” Fuel 160 (2015): 479-489.
- Thiophene mitigates high temperature fouling of metal surfaces in oil refining.” Fuel 139 (2015): 411-424
- Corrosion-fouling of 316 stainless steel and pure iron by hot oil.” Energy & Fuels 25.10 (2011): 4540-4551